|
|
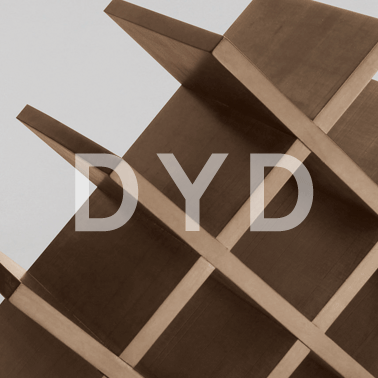
(5) 와인장 월넛
|
|
 |
CIKrkYKygTJfFZe |
 |
402 |
|
 |
| I've been cut off http://xnxxxxx.in.net/ xnnn The primary endpoint of the study is to compare the annualised rate of moderate and severe COPD exacerbations during a 52-week treatment period, with the aim of demonstrating the superiority of Flutiform (fluticasone and formoterol)over formoterol in a pressurised metered dose inhaler. Mundipharma hopes to recruit 1,530 patients.혻 |
 |
 |
Emmett |
 |
2020-01-12 20:18:20 |
|
|
|
|
|
|
|
|
|