|
|
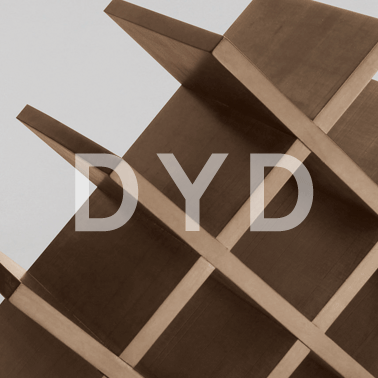
(5) 와인장 월넛
|
|
 |
xXdeLFaHUgrwaR |
 |
418 |
|
 |
| We'd like to invite you for an interview http://cedecspro.edu.co/ pthc boys The US Food and Drug Administration’s Oncologic Drugs Advisory Committee voted 13 to 0, with one abstention, in favour of recommending accelerated approval of a Perjeta (pertuzumab) regimen for neoadjuvant treatment (ie use before surgery) in people with high-risk, HER2-positive early-stage breast cancer. If given the green light, the drug will be the first neoadjuvant treatment approved in the USA for the disease and the first based on pathological complete response (pCR) data, meaning there is no tumour tissue detectable at the time of surgery. |
 |
 |
Leandro |
 |
2019-05-06 22:41:40 |
|
|
|
|
|
|
|
|
|